Acid Is A Proton Donor
5.one: Brønsted–Lowry Acids and Bases
- Page ID
- 28108
In 1923, chemists Johannes Brønsted and Martin Lowry independently developed definitions of acids and bases based on compounds abilities to either donate or have protons (H+ ions). Here, acids are defined equally beingness able to donate protons in the form of hydrogen ions; whereas bases are defined as being able to accept protons. This took the Arrhenius definition one footstep further as water is no longer required to be present in the solution for acid and base of operations reactions to occur.
Brønsted-Lowery Definition 
J.N. Brønsted and T.M. Lowry independently developed the theory of proton donors and proton acceptors in acrid-base reactions, coincidentally in the aforementioned region and during the same yr. The Arrhenius theory where acids and bases are defined by whether the molecule contains hydrogen and hydroxide ion is as well limiting. The main effect of the Brønsted-Lowry definition is to identify the proton (H+) transfer occurring in the acrid-base reaction. This is best illustrated in the post-obit equation:
\[HA+Z \rightleftharpoons A^− + HZ^+\]
| Acrid | Base | |
|---|---|---|
| Donates hydrogen ions | Accepts hydrogen ions. | |
| HCl+ | HOH → | HiiiO+ + Cl- |
| HOH+ | NH3→ | NH4 + + OH- |
The determination of a substance as a Brønsted-Lowery acid or base of operations tin can merely be done by observing the reaction. In the instance of the HOH it is a base of operations in the first case and an acid in the second case.
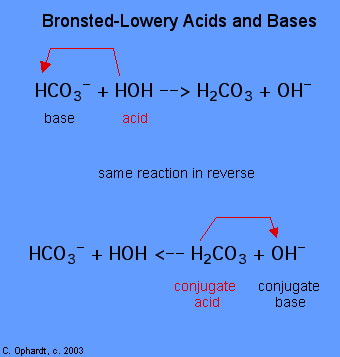
To determine whether a substance is an acrid or a base, count the hydrogens on each substance before and after the reaction. If the number of hydrogens has decreased that substance is the acid (donates hydrogen ions). If the number of hydrogens has increased that substance is the base (accepts hydrogen ions). These definitions are ordinarily applied to the reactants on the left. If the reaction is viewed in reverse a new acrid and base tin be identified. The substances on the right side of the equation are called conjugate acid and conjugate base compared to those on the left. Also note that the original acid turns in the conjugate base later the reaction is over.
Acids are Proton Donors and Bases are Proton Acceptors 
For a reaction to be in equilibrium a transfer of electrons needs to occur. The acid will give an electron abroad and the base of operations will receive the electron. Acids and Bases that work together in this fashion are called a cohabit pair made up of conjugate acids and cohabit bases.
\[ HA + Z \rightleftharpoons A^- + HZ^+ \]
A stands for an Acidic compound and Z stands for a Basic chemical compound
- A Donates H to grade HZ+.
- Z Accepts H from A which forms HZ+
- A- becomes cohabit base of HA and in the reverse reaction it accepts a H from HZ to recreate HA in order to remain in equilibrium
- HZ+ becomes a conjugate acid of Z and in the reverse reaction information technology donates a H to A- recreating Z in society to remain in equilibrium
Questions
- Why is \(HA\) an Acid?
- Why is \(Z^-\) a Base of operations?
- How can A- be a base when HA was and Acrid?
- How tin HZ+ be an acrid when Z used to be a Base?
Now that we sympathize the concept, let's look at an an example with actual compounds!
\[HCl + H_2O \rightleftharpoons H_3O^+ + Cl^¯\]
- HCL is the acrid because it is donating a proton to H2O
- HiiO is the base considering H2O is accepting a proton from HCL
- HthreeO+ is the conjugate acid because it is donating an acrid to CL turn into it's conjugate acid HiiO
- Cl¯ is the conjugate base because it accepts an H from H3O to render to it's conjugate acid HCl
How can HtwoO be a base? I idea it was neutral?
Answers 
- It has a proton that can be transferred
- It receives a proton from HA
- A- is a cohabit base because it is in need of a H in order to remain in equilibrium and return to HA
- HZ+ is a cohabit acrid because it needs to donate or give away its proton in lodge to return to it'south previous state of Z
- In the Brønsted-Lowry Theory what makes a chemical compound an element or a base is whether or not it donates or accepts protons. If the H2O was in a different trouble and was instead donating an H rather than accepting an H it would be an acid!
Conjugate Acid–Base Pairs 
In aqueous solutions, acids and bases can be defined in terms of the transfer of a proton from an acrid to a base. Thus for every acidic species in an aqueous solution, there exists a species derived from the acid by the loss of a proton. These two species that differ by but a proton found a conjugate acid–base pair.
All acrid–base reactions contain two cohabit acid–base pairs.
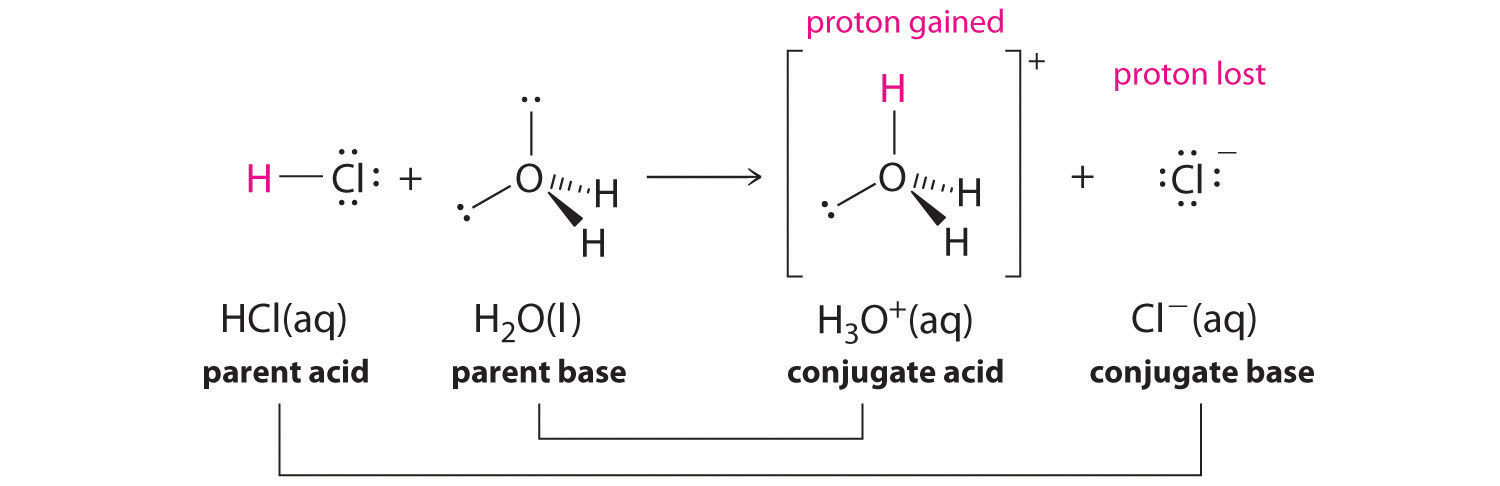
For example, in the reaction of HCl with water, HCl, the parent acrid, donates a proton to a water molecule, the parent base, thereby forming Cl-. Thus HCl and Cl- establish a conjugate acid–base of operations pair. By convention, nosotros always write a conjugate acrid–base pair as the acid followed by its conjugate base. In the reverse reaction, the Cl- ion in solution acts as a base to accept a proton from H2O and HCl . Thus H3O+ and H2O found a 2d conjugate acid–base pair. In general, whatsoever acid–base reaction must incorporate two conjugate acid–base pairs, which in this case are HCl/Cl- and H3O+/H2O.
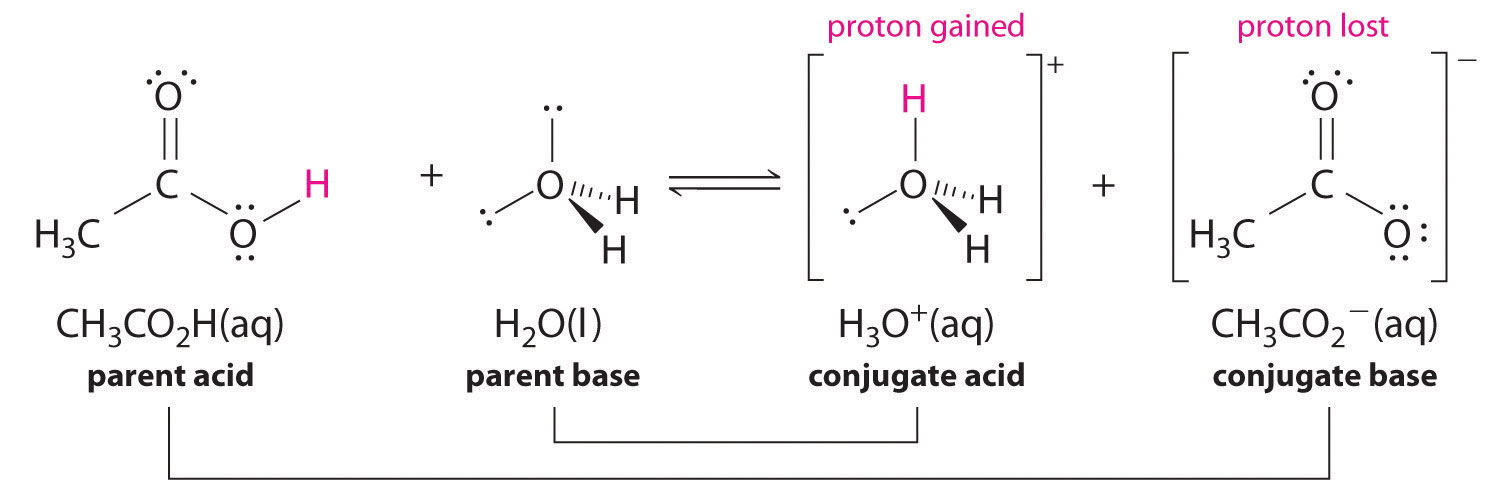
Similarly, in the reaction of acerb acrid with water, acerb acid donates a proton to h2o, which acts as the base of operations. In the reverse reaction, \(H_3O^+\) is the acid that donates a proton to the acetate ion, which acts equally the base of operations. One time over again, we accept two conjugate acid–base pairs: the parent acrid and its conjugate base (CHthreeCO2H/CH3COtwo - ) and the parent base and its conjugate acrid (H3O+/H2O).
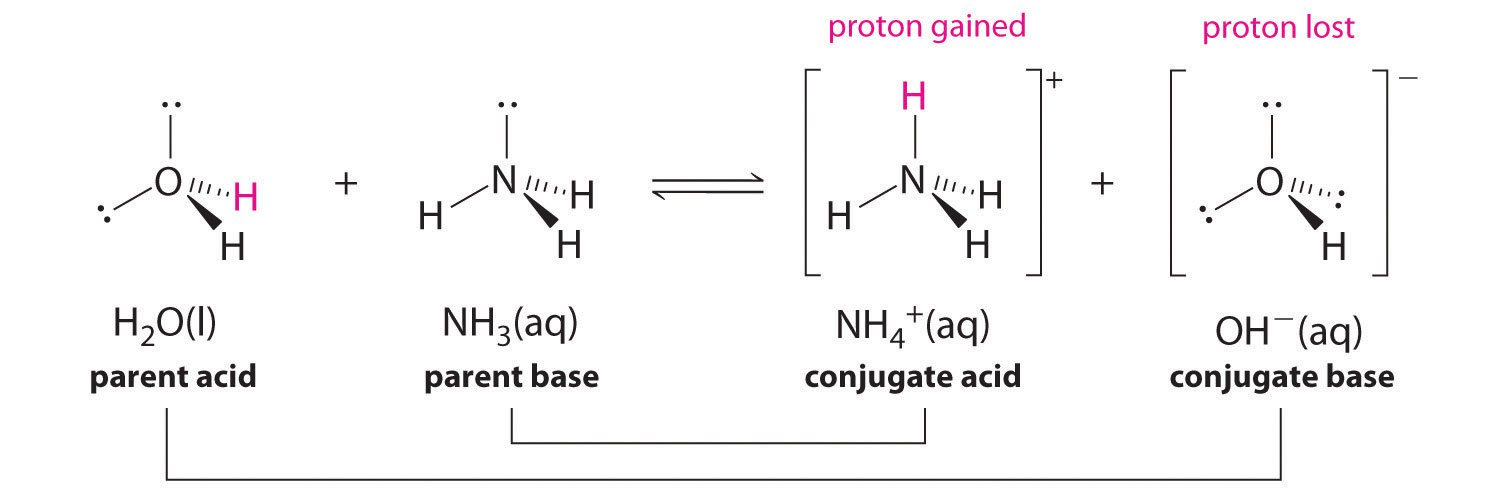
In the reaction of ammonia with water to give ammonium ions and hydroxide ions (Figure 16.3), ammonia acts as a base by accepting a proton from a water molecule, which in this example means that water is interim as an acrid. In the contrary reaction, an ammonium ion acts as an acrid by donating a proton to a hydroxide ion, and the hydroxide ion acts as a base of operations. The cohabit acid–base pairs for this reaction are NH4 +/NH3 and HiiO/OH-.
Some common conjugate acrid–base pairs are shown in Effigy \(\PageIndex{four}\).

Acid Is A Proton Donor,
Source: https://chem.libretexts.org/Courses/University_of_Illinois_Springfield/UIS%3A_CHE_267_-_Organic_Chemistry_I_(Morsch)/Chapters/Chapter_02%3A_Acids_and_Bases/5.1%3A_Br%C3%B8nsted%E2%80%93Lowry_Acids_and_Bases#:~:text=reaction%20is%20over.-,Acids%20are%20Proton%20Donors%20and%20Bases%20are%20Proton%20Acceptors,base%20will%20receive%20the%20electron.
Posted by: calderonmisiongs71.blogspot.com


0 Response to "Acid Is A Proton Donor"
Post a Comment