An Atom Of Bromine-79 Contains
Chemguide: Cadre Chemistry 14 - 16
Subatomic Particles, the Nucleus and Isotopes
This page looks briefly at the three subatomic particles nosotros talk about at this level (protons, neutrons and electrons), and and so goes on to look at how you lot work out the numbers of protons and neutrons in the nucleus. Information technology finishes by looking at the beingness of isotopes of elements.
Protons, neutrons and electrons
The table summarises the key facts well-nigh these particles.
| relative mass | relative charge | |
| proton | 1 | +1 |
| neutron | one | 0 |
| electron | 1/1836 | -1 |
What you lot need to observe:
-
Most of the mass of an cantlet is due to the protons and neutrons. The mass due to the electrons is negligibly pocket-sized past comparison.
-
A proton carries a positive charge; an electron carries an equal negative charge. Neutrons are neutral - they accept no accuse.
The nucleus
The nucleus is at the middle of the atom and contains the protons and neutrons. Protons and neutrons are collectively known equally nucleons. You aren't very likely to meet this term at this introductory level.
Virtually all the mass of the atom is concentrated in the nucleus, because the electrons weigh so little.
Working out the numbers of protons and neutrons
No of protons = ATOMIC NUMBER of the cantlet
The diminutive number is besides given the more descriptive proper noun of proton number , and is related to the position of the element in the Periodic Table.
No of protons + no of neutrons = MASS NUMBER of the atom
The mass number is as well called the nucleon number - again yous probably won't meet that at this level.
Using fluorine as an example, this information can be given merely in the form:
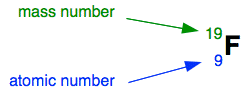
How many protons and neutrons has this atom got?
-
The atomic number counts the number of protons (nine).
-
The mass number counts protons + neutrons (nineteen).
-
If in that location are 9 protons, in that location must be 10 neutrons for the total to add up to xix.
The diminutive number is tied to the position of the element in the Periodic Table, and then the number of protons defines what sort of element you are talking about. So if an atom has 8 protons (atomic number = eight), information technology must be oxygen. If an cantlet has 12 protons (atomic number = 12), information technology must be magnesium.
Similarly, every argon atom (atomic number = 18) has 18 protons; every uranium atom (atomic number = 92) has 92 protons.
Some other example - working out the number of protons and neutrons in Br-79
Br-79 is another way of writing 79Br. So bromine-79 is a bromine atom with a mass number of 79.
Beginning you demand to find bromine, Br, in the Periodic Table. You actually need a printed version of this, and will discover a simple downloadable version from this site.
-
Bromine has an atomic number of 35, and and so has 35 protons.
-
You lot are told that the mass number is 79, then there are a total of 79 protons plus neutrons.
-
Therefore there must be (79 - 35) neutrons = 44 neutrons.
Some warnings!
Periodic Tables will unremarkably give you two numbers for each element. The atomic number is the smaller number - don't get the two numbers confused.
The other number is Non the mass number - it is the relative atomic mass of the element, which in most cases won't be the same as the mass number.
In a Periodic Table such as the ane I take suggested, which gives the relative atomic mass to one decimal identify, information technology is obvious that a number similar 79.9 (which is given for bromine in this tabular array) tin can't exist the mass number. The total number of protons plus neutrons has to be a whole number.
Except for i common example which you lot might be expected to remember, in an test you would unremarkably be given the mass number of the cantlet if you need information technology - equally I have done at the top of this example.
We will talk some more well-nigh relative atomic mass in a moment.
Isotopes
The number of neutrons in an atom can vary inside modest limits. For example, there are three kinds of carbon cantlet 12C, 13C and 14C. They all have the same number of protons, but the number of neutrons varies.
| protons | neutrons | mass number | |
| carbon-12 | 6 | vi | 12 |
| carbon-13 | 6 | seven | 13 |
| carbon-14 | six | 8 | 14 |
These different atoms of carbon are called isotopes . The fact that they have varying numbers of neutrons makes no difference whatever to the chemical reactions of the carbon.
Isotopes are atoms which have the same atomic number but unlike mass numbers. They have the same number of protons but unlike numbers of neutrons.
Isotopes and relative diminutive mass
This isn't intended to be a proper introduction to relative atomic mass - merely a brief look at how it relates to isotopes.
An case using chlorine
This is the example where the numbers are so easy that you might well be expected to remember them.
Chlorine has two isotopes, Cl-35 and Cl-37, and ordinary chlorine contains these in the ratio of 3 atoms of Cl-35 to every i atom of Cl-37 (to a proficient-enough approximation for our purposes).
If you accept a sample of chlorine it will contain unbelievably vast numbers of chlorine atoms, and it is useful to be able to give an average value for the mass of a chlorine atom. An boilerplate of 35 and 37 is 36, just that doesn't let for the fact that there are three times as many Cl-35 atoms as Cl-37.
Relative atomic mass is a weighted average (often called a weighted mean) of the masses of the isotopes. That is an average which takes account of the different proportions of the diverse isotopes.
Suppose you had four typical chlorine atoms - 3 atoms of Cl-35 and 1 cantlet of Cl-37.
The total mass of the iv atoms would exist (3 ten 35) + (i ten 37) = 142
The "boilerplate" mass of one atom is therefore 142/iv = 35.five
If you lot look at the Periodic Tabular array yous will notice that 35.5 is the figure quoted as the relative atomic mass of chlorine.
In case you are wondering almost the units of relative diminutive mass - there aren't whatsoever! It is measured on a scale on which the carbon-12 isotope has a mass of exactly 12 units. Yous don't demand to worry about that at the moment.
Note:You will notice a proper give-and-take of relative atomic mass in the Calculations department of this site on this page.
An Atom Of Bromine-79 Contains,
Source: https://chemguide.co.uk/14to16/atoms/nucleus.html
Posted by: calderonmisiongs71.blogspot.com


0 Response to "An Atom Of Bromine-79 Contains"
Post a Comment