Addition Substitution And Elimination Reactions
Add-on Reactions of Alkenes
In previous series, we've discussed acid-base reactions, nucleophilic substitution reactions, and elimination reactions. These represent three of the four most of import reaction types in a typical Org one course.
What each of these reactions have had in common so far is that each of them begin with a Lewis base (which we call a "base" or "nucleophile" depending on whether information technology is attacking hydrogen or carbon) donating a lonely pair to an electrophile (either hydrogen or carbon).
In this serial of posts we'll cover the fourth major form of reactions, addition reactions. As we'll see, what makes this class of reactions dissimilar is that a double bond (a π-bond, to be more specific) that will act as the electron-pair donor.
In other words, we'll see thatπ-bonds tin can be nucleophiles likewise!
Furthermore, since by definition π-bonds bridge 2 carbons, we shall encounter that this class of reactions will take interesting ramifications concerning the identity of the products ("regioselectivity" and "stereoselectivity", respectively) that we will cover in subsequent posts.
And then let's showtime, shall we?
Table of Contents
- Iii Reactions of Alkenes – Don't Worry About "Why", Nevertheless, Only Focus On The Bonds That Form And Interruption
- The Key Pattern Of All These Reactions Is That They Break A C–C Pi Bond And Form Two Single Bonds On Adjacent Carbons (The Exact Reverse Of Emptying)
- The General Pattern For Add-on Reactions
- Summary: Add-on Reactions
1. Three Reactions of Alkenes – Don't Worry About "Why", Nonetheless, Just Focus On The Bonds That Form And Suspension
I e'er remember it's important to describe the "what" before we get to the "why" or "how". Earlier nosotros tin can sympathise how or why something happens, it's important to be able to merely recognize the essential design. And equally always, this will come up from what experimental observations tell usa.
Allow'southward look at an experimental observation that dates back well over 140 years. In the belatedly 1860's, the Russian pharmacist Vladimir Markovnikov made the post-obit observation: alkenes treated with hydrobromic acid formed alkyl bromides.
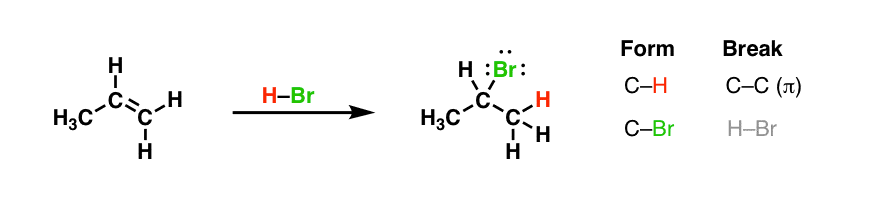
Note the design of bail-forming and bail-breaking here: we're breaking a C-C π bond and forming a C-Br and C-H bail on next carbons.
Here's another example. In the late 1800's it was discovered by French pharmacist Paul Sabatier that when alkenes are treated with hydrogen gas in the presence of finely divided nickel, the following reaction occurs:
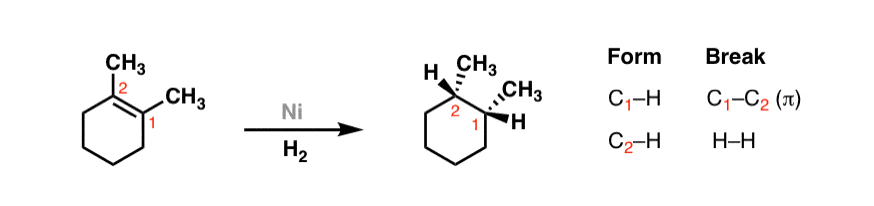
Sabatier won the 1912 Nobel Prize in chemistry for the development of this reaction, which was subsequently constitute to occur with many dissimilar varieties of metallic catalysts also nickel, including palladium, platinum, and many other "late" metals.
Again, annotation the pattern: breaking a C–C π bail and forming two C–H bonds on adjacent carbons. [Don't worry so much about the dashes and wedges for at present – we'll get there in a after post].
Here's ane last example. If you take an alkene (like cyclohexane, for instance) and add together elemental (liquid) bromine, the following reaction occurs:
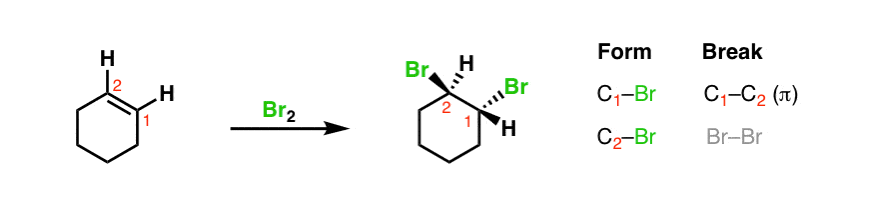
ii. The Key Design Of All These Reactions Is That They Break A C–C Pi Bail And Class Two Single Bonds On Adjacent Carbons (The Exact Contrary Of Emptying)
Again, note the pattern – pause C–C π [and Br-Br] and course C–Br bonds on adjacent carbons. [Nosotros'll deal with the dashes and wedges in subsequent posts – information technology'southward OK to just ignore them for at present].
If you lot've got a really good memory, you might notice that this pattern is strangely familiar. If nosotros go waaay back into the archives, we've seen a reaction that fits this pattern exactly…. butin reverse!It's our former friend the emptying reaction!
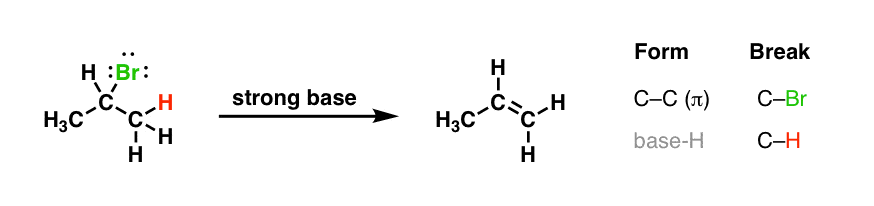
[I've left the "strong base" here as generic, simply a typical example would be NaOCHthree or NaOCHtwoCHiii]
As we've previously seen, emptying reactions involve breaking ii single bonds on adjacent carbons and forming a new C–C π bond. Notice how these two reactions (addition and elimination) achieve the exact reverse results here.
- In the add-on reaction [the first reaction at the top of the page] , we're forming C-Br and C-H, and breaking C–C π [nosotros're also breaking H-Br]
- In the elimination reaction, we'rebreakingC–Br and C–H, andforming C–C π [and forming a bond between the base of operations and hydrogen]
3. The General Pattern For Add-on Reactions
We can fifty-fifty generalize these patterns beyond this specific example of H-Br. Likewise, for improver reactions, the general pattern looks like this:
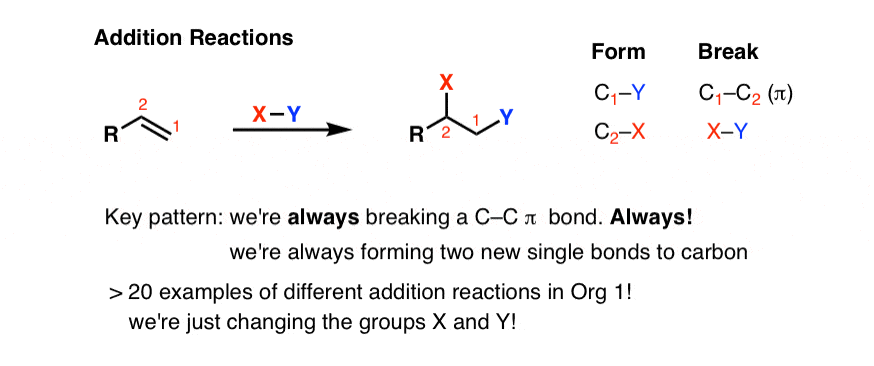
As we'll see , there are many, many more examples of addition reactions we'll see beyond the 3 examples nosotros've seen here. Only they all follow the same essential pattern. We'll e'er break a C-C π bond and we'll e'er be forming ii new unmarried bonds to carbon.
4. Summary: The General Pattern For Addition Reactions
Lots of mysteries remain for us, even so: for instance, did you observe in the first instance that Br added to the most substituted carbon? And how in the second, the two hydrogens added to the aforementioned side of the alkene, but in the tertiary, the bromines added to contrary sides? We'll go through these patterns in more detail in subsequent posts.
NEXT Mail service: Addition Reactions – Regioselectivity
Addition Substitution And Elimination Reactions,
Source: https://www.masterorganicchemistry.com/2013/01/22/addition-reactions-the-opposite-of-elimination/
Posted by: calderonmisiongs71.blogspot.com


0 Response to "Addition Substitution And Elimination Reactions"
Post a Comment